Overview
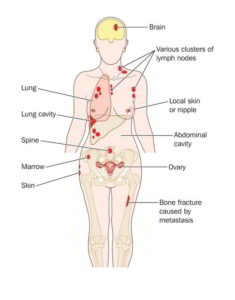
Nasopharyngeal carcinoma is a malignant tumor that affects the head and neck. Typically, this cancer starts in the upper part of the throat and spreads from there.
Unfortunately, metastatic cases of nasopharyngeal carcinoma are very challenging to manage. However, there is a new drug in the bloc – Toripalimab, which was just granted a breakthrough therapy designation by the United States Food and Drug Administration (FDA) for the treatment of recurrent or metastatic cases of nasopharyngeal carcinoma.
In this article, we will cover some basic information about Toripalimab, then dissect the results of phase III clinical trial.
What is Toripalimab?
Toripalimab is a monoclonal antibody that acts against a specific type of receptor known as programmed cell death 1 (PD-1). This action yields an inhibitory for cellular growth, which halts the proliferation of cancer.
The stimulation of PD-1 is responsible for the suppression of T-cell activity, which is how cancer gets around your immune system. Researchers refer to this process as tumor evasion from host immunity.
The good news is that Toripalimab blocks the activation of PD-1, which gives T cells a boost. As a result, they become more efficient at identifying and neutralizing cancer cells.
The results of phase III clinical trial (NCT03581786) in advanced nasopharyngeal carcinoma patients
The FDA recently granted a breakthrough therapy designation for Toripalimab combined with gemcitabine and cisplatin as first-line therapy for:
Recurrent and/or metastatic nasopharyngeal carcinoma
This designation is due to the findings of phase III clinical trial, which showed the effectiveness of this combination in improving progression-free survival (PFS, which is the time it takes for the cancer to stop responding to the treatment) and survival (OS) rate.
The randomized study included 289 participants, who were divided into:
- Group one (146 participants) – received Toripalimab (240 milligrams) plus gemcitabine (1000 mg/m2) and cisplatin (80 mg/m2) on day 1 every 3 weeks for 6 cycles. After the cycles were complete, researchers gave participants a maintenance therapy that consisted of 240 mg of Toripalimab every 3 weeks.
- Group two (143 participants) – received gemcitabine and cisplatin plus a placebo every 3 weeks for 6 cycles. The maintenance scheduling was similar to group one. However, instead of giving participants Toripalimab, they gave them a Placebo.
As you can see, the primary difference between the two groups resides within giving participants Toripalimab or a Placebo. Therefore, the clinical results of the two groups will allow to determine the efficacy of the former.
The key difference between the two groups was the PFS, which was in favor of Toripalimab.
Additionally, the 1-year survival rate for the first group was 91.6% compared to 87.1% in the second group. That means more patients in the Toripalimab were alive after 1 year.
The gap of the 2-year survival rate was even more noticeable:
- The Toripalimab group – 77.8%
- The Placebo group – 63.3%
As for side effects, both groups experienced similar symptoms.
Note that the FDA has previously granted breakthrough therapy designation for Toripalimab monotherapy to treat recurrent or metastatic nasopharyngeal carcinoma.
Takeaway message for nasopharyngeal carcinoma patients
The combination of Toripalimab with gemcitabine and cisplatin could generate impressive results in treating recurrent and/or metastatic nasopharyngeal.
Hopefully, the upcoming few months will let us know more about the incorporation of this combo in practice.
Talk to us so see if we can help you to actually get the most advanced treatments
TRIAL•IN Pharma
Because we, do not give up on life!
Contact us 24/7 –
Call center +44.2082.426.039