Background
The antibody-drug conjugate Trodelvy offers statistically and clinically significant benefits for patients with heavily pretreated HR+/HER2- breast cancer, according to results of the TROPiCS-02 study, recently presented.
HR+/HER2- is defined as hormone receptors positive – ER and PR positive – and HER2 status negative breast cancer.
Heavily pretreated patients are patients who have failed at least two prior chemotherapy regimens in this study.
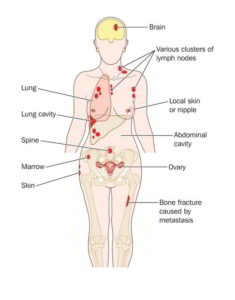
Breast cancer is a leading cause of cancer death, and HR+/HER2- disease is the most common subset, representing approximately 70% of this disease. For metastatic, HR+ breast cancer, international guidelines recommend sequential endocrine therapy, starting with first-line endocrine drug, like Tamoxifen, Letrozole and others given in combination with CDK4/6 inhibitor drugs, like Verzenio, Ibrance and Kisqali.
When resistance to endocrine therapy develops, single-agent chemotherapy is recommended but is associated with declining efficacy and increased toxicity.
So, in later lines of treatment there are limited options to offer patients.
What is Trodelvy?
Trodelvy is directed to Trop-2. Trop-2 is a biological entity is overexpressed in many cancers, including breast cancer, and is associated with poor survival. About 80% of breast cancer tumors highly express Trop-2.
It is currently approved for use in metastatic Triple-Negative Breast Cancer patients who have received at least 2 prior therapies, with at least 1 given in the metastatic setting.
About the TROPiCS-02 Study in Breast Cancer
The goal of this phase III study was to determine whether Trodelvy can help fulfill the clinical need in HR+/HER2-, in which patients were randomized to receive Trodelvy or treatment of physician’s choice (Xeloda, Halaven, Navelbine, or Gemzar) until disease progression or unacceptable toxicity.
All patients had received 2 to 4 prior chemotherapy protocols.
Study Results
Trodelvy was associated with better median PFS (Progression Free Survival, which is the length of time until progression of the disease or unacceptable toxicity) vs physician’s choice: 5.5 versus 4.0 months.
The percentage of patients with no disease progression or unacceptable toxicity at 6-month was 46% versus 30% and at 12-month was 21% versus 7%, in favor of Trodelvy.
In the first of three planned OS analyses, the median Survival was 13.9 months with Trodelvy versus 12.3 months with physician’s choice therapy.
The percentage of patients who experienced decrease in the volume of their tumors was 21% versus 14% in favor of Trodelvy.
The percentage of patients who experienced decrease in the volume of their tumors plus stable disease (no change) was 34% versus 22% in favor of Trodelvy.
Overall, 74% in the Trodelvy arm versus 60% of patients in the physician’s choice arm experienced harsh side effects.
Unfortunately, there was 1 treatment-related death in the Trodelvy arm and none in the treatment of physician’s choice arm.
Key Messages for Breast Cancer patients
- Trodelvy demonstrated PFS benefit over chemotherapy in
- A higher proportion of patients were alive and progression-free
- Trodelvy also demonstrated an overall health-related quality of life benefit over chemotherapy, with delayed deterioration in fatigue.
Conclusion
Trodelvy demonstrated significant and clinically meaningful benefit and should be considered a potential treatment option in this heavily pretreated population of HR+/HER2- breast cancer.
Talk to us so see if we can help you to actually get the most advanced treatments
TRIAL•IN Pharma
Because we, do not give up on life!
Contact us 24/7 –
Call center +44.2082.426.039
Read more about breast cancer >>