Background
Patients with advanced or metastatic kidney cancer are a population that despite recent advances remains in need of additional therapeutic options that extend survival.
The immunotherapy and tyrosine kinase inhibitor combination of Opdivo and Cabometyx combination may become a new first-line option for patients with metastatic stage 4.
About the CheckMate-9ER Study
The open-label, global phase III CheckMate-9ER trial randomized 701 patients with previously untreated advanced or metastatic kidney cancer patients in a 1:1 ratio to frontline Opdivo plus Cabometyx or Sutent.
The FDA approved Cabometyx in December 2017 for patients with advanced disease.
The FDA approved the Opdivo in November 2015 for patients with metastatic disease.
Results from Preliminary Analysis Data
In comparison to Sutent, Opdivo combined with Cabometyx:
- Prolonged survival by lowering the risk of death by 40%
- Eeduced the risk of disease progression under the treatment by 50%
- Improved percentage of patients who had their disease shrunken or stopped from further growing.
There were no new side effects with any of the study drugs which are already approved.
The best treatment for a cancer patient is to get the most advanced cancer drugs in advanced stages of development. There, the hope and the chance to extend life go far beyond the standard protocols.
Contact us to find out what is the best treatment for YOU
TRIAL•IN Pharma
Because we, do not give up on life!
Contact us 24/7 –
Call center +44.2082.426.039
For further reading about the results>>
For further reading about Kidney Cancer>>

About Kidney Cancer
Kidney cancer, or Renal cancer, begins when some of the cells that make up the inside of the kidney begin to grow uncontrollably. This abnormal growth creates a tumor, which is cancer.
The kidneys are a pair of organs that belong to the secretory system and are located in the back of the abdominal cavity. The key role of the kidneys is blood filtration, balancing the levels of salts in the body and body fluids as well as hormone production.
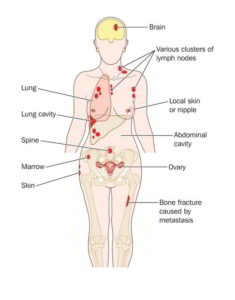
There are types of kidney cancer that grow slowly and even require little or no treatment, and there are aggressive types that can rapidly spread to other organs in the body and create metastases. This condition is defined as “metastatic kidney cancer” or “stage 4 kidney cancer.”
To improve patients’ chances, the American Society for Clinical Oncology, the ASCO, that includes various specialists in its panel, such as oncologists and radiologists, guides oncologists to inform cancer patients in all topics related to clinical trials in every stage of their disease.
The National Cancer Institute, the NCI, of the United States, stresses that for kidney cancer patients, joining one of the many clinical trials around the world might increase the chances of treatment success over the standard protocols.
Types of Kidney Cancer
- Renal cell carcinoma, RCC, in most cases, in 90% of cases.
- Tumor transplantation cells, for example, the Transient Cell Cancer, TCC, are present in about 10% of cases.
- Wilms’ tumor, a type of nephroblastoma, is the most common malignant kidney tumor in children, mainly aged 15-19, and probably the source is genetic mutations, such as WT1, WT2, WTX.
Disease Causes
- Smoking
- Older people
- High blood pressure
- Exposure to chemicals
- Dialysis patients
- Genetic
Disease Symptoms
- Blood in urine
- Lower back and / or waist pain
- Fever and / or weakness
- Weight loss
- Decrease in appetite