Overview
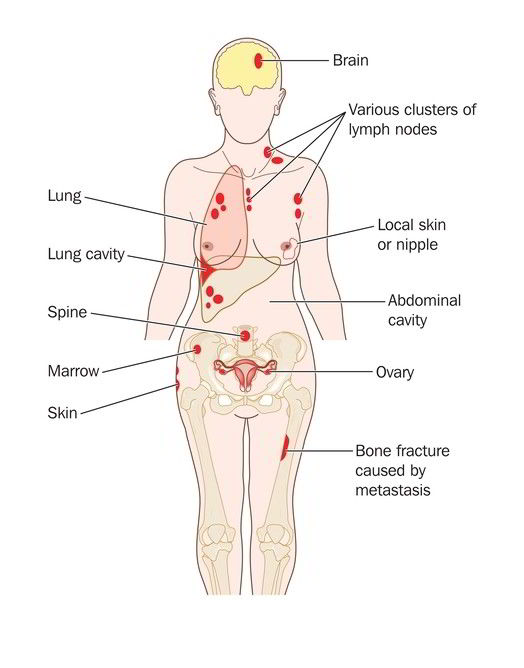
Advanced Melanoma is a challenging type of cancer to deal with, especially when it spreads to multiple sites in the body, metastasizes. Moreover, the poor response of this tumor to different chemotherapeutic agents made it challenging for doctors to treat it properly.
Unfortunately, the benign-looking shape of Melanoma makes patients consult very late. As a result, the cancer cells will usually spread before getting diagnosed.
In this article, we will cover the new evidence that showcases the effects of Relatlimab on Melanoma. But first, let us quickly define this drug and its mechanism of action.
What is Relatlimab?
Relatlimab is an immunotherapeutic agent that was developed by Bristol-Myers Squibb (BMS). Today, this treatment already proved its effectiveness in the management of Melanoma. Ongoing clinical trials are seeing whether Relatlimab helps with other cancer.
The purpose of this drug is to modify the body’s immune reaction in order to fight cancer more efficiently.
How does Relatlimab work?
Relatlimab is an anti-LAG-3 antibody. This means that it binds to the LAG-3 protein, which is present on our natural immune cells called T-cells.
The function of LAG-3 is to regulate the immune response, including activation, suppression, and growth of antitumor T-cells.
Melanoma releases some molecules that interfere with the action of LAG-3, blocking the process of T-cell proliferation. In a sense, these tumors apply the brakes to these specialized immune cells.
Relatlimab releases these brakes, stimulating T-cells to neutralize cancer cells. Consequently, your immune system becomes more active and slows down the growth of these tumors.
The effects of Relatlimab on Melanoma
In phase II/III clinical trial, researchers gave patients with advanced Melanoma two medications:
- Relatlimab
- Nivolumab – a PD-1 blocker
They assessed the effectiveness of this combination by giving a group of patients a fixed dose of Relatlimab and Nivolumab while giving a second group Nivolumab alone.
The subjects all had previously untreated metastatic or unresectable cases of Melanoma. The primary endpoint was the length of time it took for the disease to worsen, also called PFS, Progression Free Survival.
The results showed that the median PFS for the first group (i.e., patients who received Relatlimab and Nivolumab) was 10.1 months. The second group (i.e., Nivolumab alone), however, had a much lower median PFS of only 4.6 months.
At 12 months, 47.7% of the first group had not had disease progression while it was 36.0% for the second group.
This groundbreaking work demonstrated that blocking a novel immune checkpoint can be conducted safely and provide better outcomes for patients.
Speak with your oncologist for tailored medical advice about the effectiveness of Relatlimab and your eligibility to receive it.
Takeaway message for Melanoma patients
Metastatic or unresectable cases of Melanoma are extremely challenging to manage. These tumors spread across different tissues and respond poorly to medication. Fortunately, the new biological treatment gives us hope that Melanoma management will be more robust and efficient in the upcoming years.
We hope that this article managed to provide a simple explanation of Relatlimab and how it may help patients with Melanoma.
To learn more about other treatments of Melanoma, do not hesitate to check our other articles.
Talk to us so see if we can help you to actually get the most advanced treatments
TRIAL•IN Pharma
Because we, do not give up on life!
Contact us 24/7 –
Call center +44.2082.426.039