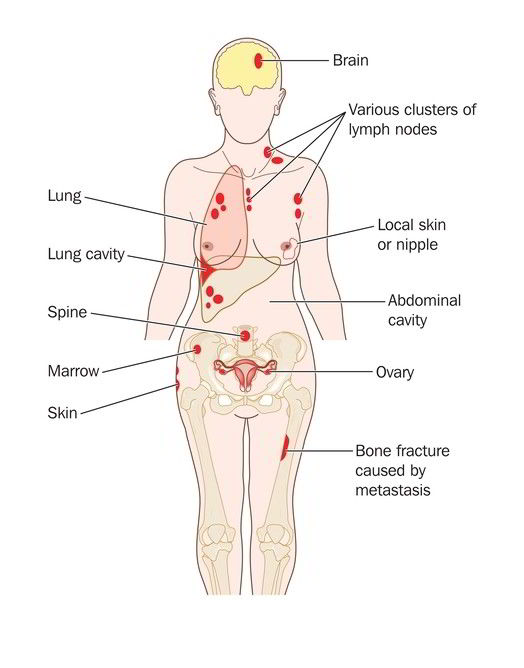
How to find innovative treatments for metastatic cancer? A short guide for stage 4 patients
Patients with metastatic cancer often encounter situations where it seems that “there is nothing more to do” but the truth is far from it.
Families and patients themselves often ask: “How to find innovative treatments for metastatic cancer?”
There are new drugs, clinical trials, personalized approaches, and technologies that are not always available through public health systems.
What to do?
Search the internet for the word “cancer” along with keywords like “new treatment,” “clinical trial,” or “recently approved drug.”
You can start with websites like MyTrial or check with companies like Trial-In Pharma.
What treatment options are worth checking?
- 🧪 Clinical trials (in Israel and abroad)
- 🚨 Drugs in compassionate use or early access programs
- 💊 Off-label treatments that are not approved for the cancer type but may still be suitable
- 🧬 Biological and targeted therapies such as ADCs, BiTE, CAR-T, TIL, TCR
- 🧫 Genetic or pathological testing like Caris, CureMatch, Exacta, and more
“At every stage where it seems there are no further options there is always something worth checking.”
Nir Erez, CRA
💡 A few numbers worth knowing
- In 2024 alone, the FDA approved 17 new oncology drugs. Read more >>
- Between 1998 and 2024, 217 new oncology drugs were approved by the FDA. Read more >>
- Out of 573 drug combinations approved between 2000–2022, 48% were targeted therapies, 43% were biological drugs, and 9% were classical treatments. Read more >>
- Over 95% of patients who turned to Trial-In Pharma received treatment recommendations that had not been previously offered to them. (Source held by company management)
Trial-In Pharma: With You on the Journey
Trial-In Pharma helps patients with metastatic cancer find advanced treatment solutions from around the world.
We work 24/7 to identify innovative and sometimes life-saving treatments that are not always available through the public system.
📞 Contact us: 072-3902977
📧 Or email us at: contact@trial-in.com