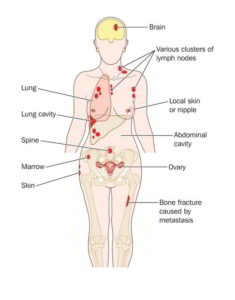
Personalized medicine has led to some dramatic successes in the treatment of cancer in patients with advanced disease. With developments in molecular diagnosis or genetic sequencing, targeted genomic-oriented drugs and therapies are used in the treatment of various cancers, especially melanoma, lung cancer, and various types of leukemia.
However, there remains an open question as to how many advanced or metastatic cancer patients, stage 4, really benefit from genetic screening tests. It turns out that almost nothing, as of today.
A number of cancer researchers from one of the world’s leading cancer centers, Memorial Sloan Kettering in New York, have examined this issue in depth and raised several parameters that affect the outcome of the treatment:Heterogeneity among patients [there are no two identical cancer patients, even if they have the same type of disease], the technique used for the genetic examination, the drugs that were ultimately selected for treatment following the genomic diagnosis, and whether the drugs that come up as an option from the report are accessible to the patients.
A group of researchers from the Health & Sciences University, OSHU, examined the percentage of patients eligible for genetic testing who are also candidates for genomic-oriented therapies from January 2006 to January 2018, and the percentage of patients who actually benefited from these treatments.
OSHU researchers found that the percentage of patients screened for genetic diagnosis for genome-directed therapy increased consistently from 5.09% in 2006 to 8.33% in the US. The authors attributed this increase to FDA approval of targeted drugs for stage 4 melanoma and metastatic lung cancer and AML.
When the definition of genome-oriented therapies was expanded, an increase was noted from 10.5% to 15.44%.
However, the researchers estimated that a small percentage of cancer patients did benefit from genome-targeted therapies and drugs following the genetic test
Note 
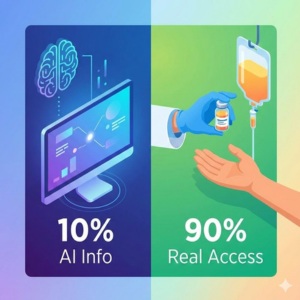
Of all Americans expected to die of cancer in 2018 (90% die of stage 4 metastatic disease), only 4.9% will respond to treatments based on genetic testing, at best! And this is another assumption that 100% of the population is being tested, receives the drugs at no cost and other optimistic assumptions, so that the real percentage is lower than 4.9%.
As of today, if you do not have lung cancer or melanoma and possibly ovarian cancer, chances are that these genetic tests will not bring you real value in practice.
One of the reasons for the low numbers is that there are still many mutations that can be diagnosed but there is no drug that works on them or that there are mutations that the tumor microenvironment itself, within the lesion itself, affects its susceptibility to the same drugs that are already there. For example, a drug targeted to a BRAF mutation has shown in various cancer studies that in different cancers in which this mutation has been diagnosed, response rates vary.
Next-Generation Sequencing, NGS
There is a debate in the oncology world about whether a genomic diagnosis (also called genetic sequencing or molecular / genetic testing) is necessary for cancer patients with metastatic disease, metastatic cancer stage 4. There is no consensus in this world and if it is not agreed, it’s probably not a necessity.
It turns out that world-renowned cancer experts recommend these tests for metastatic cancer patients – note! – Only after (!) the patient has exhausted all the therapeutic options, in order to see whether it will be possible to find something else in the end of his life..
A study called IMPACT began in 2007 and analyzed the impact of personalized medicine based on genetic testing on patients in: melanoma, gastrointestinal cancer (gastric cancer, gastric cancer, etc.), gynecological cancer, breast cancer, lung cancer and thyroid cancer, who have exhausted all treatment options for their disease – some of them even received 16 previous treatments!
15% percent of the patients who received a drug following the genomic diagnosis remained alive after 3 years of treatment, compared with 7% of patients who did not receive the drug following the diagnosis. 6% of the patients who received treatment after the diagnosis survived 10 years later than 1% of those who did not receive the drug following the diagnosis.
Bottom line, most of stage 4 metastatic patients do not respond to the drugs listed in the NGS report, and as of today, these tests do not bring them value at the end of the day, their illness worsens and their condition deteriorates.
So where are we, Trial-In-Pharma, in the picture?
It should be noted that the vast majority of patients receiving a drug based on the IMPACT genomic diagnosis received new drugs in the framework of clinical trials. Very few patients receive approved drugs based on genetic testing.
Currently, there are no approved drugs in the world for most of the mutations. Most of these innovative and groundbreaking drugs are in research, and only those who participate and are suitable for trials with these drugs have a higher chance of prolonging their lives. Incidentally, genetic testing is free for anyone who participates in these studies. The research team finances and does the testing itself in its laboratory.
To read more:
https://www.targetedonc.com/…/quantifying-the-benefits-of-p…